Abstract
Background. CLL-like monoclonal B-cell lymphocytosis (MBL) is considered a requisite precursor of CLL. Since MBL is also associated with inflammatory disorders, other cancers, and increased incidence of infections (Landgren et al, 2007; Moreira et al, 2013; Solomon et al, 2016), an underlying immune alteration might be at the root of the condition's onset and progression to CLL. Patients with CLL whose clone expresses mutated (M-CLL) and unmutated (U-CLL) IGHV genes differ in inflammatory profiles, with the latter exhibiting a more activated immune system, in line with the distinct capabilities of the two CLL subtypes to interact with the microenvironment with M-CLL being more anergic. Here, we studied the serum protein profiles in MBL, CLL patients, and age-matched healthy donors (HD). Since a sufficient number of MBL subjects with unmutated IGHV was not available, only MBL with mutated IGHV (M-MBL) and M-CLL patients were analyzed.
Objectives. 1. Compare serum proteomic profiles of the three subsets of individuals. 2. Identify protein clusters more restricted to M-MBL.
Methods. In all, 34 subjects were studied: 10 M-MBL, 12 M-CLL, and 12 HD. All individuals were treatment naive at sample collection, and all MBL subjects remained stable over a median follow-up of 53 months. Serum protein levels were quantified using a library of modified single-stranded DNA aptamers (SOMAmers) that measure 1,310 protein targets (SomaLogic). P-values <0.05 were required to consider protein levels as differentially expressed (DE) between groups. The protein-specific STRING database v11.5 was used for analyses. Functional enrichments were selected according to i) confidence > 0.4; ii) lowest significant (< 0.05) FDR P-values; iii) network description, focusing only on informative disease-related pathways. The Markov Cluster (MCL) algorithm was used to identify clusters of non-redundant, upregulated proteins related to functionally-associated networks. Serum levels of selected molecules were validated using the U-PLEX Platform (MSD) in 24 HD, 29 M-MBL and 25 M-CLL.
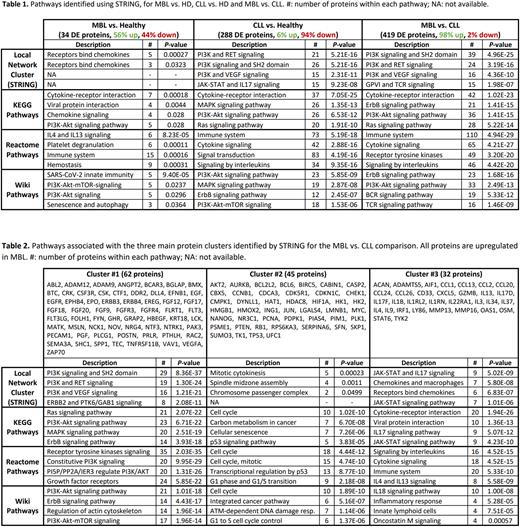
Results: Levels of 34 proteins were DE in MBL vs. HD [19 upregulated (u), 56%; and 15 downregulated (d)]; 288 in CLL vs. HD [16u, 6%; and 272d]; and 419 in MBL vs. CLL [411u, 98%; and 8d]. U-PLEX assays validated the significantly increased levels of IL8 (P = 0.020) and CXCL11 (P = 0.038) in MBL vs. HD and IL4 (P = 0.029) in MBL vs. CLL.
STRING analysis of the DE proteins between groups is shown in Table 1. Pathways related to viral infections were only highlighted in MBL vs. HD. Cytokine activity, receptor signaling, and the PI3K-Akt pathway that promotes proliferation, were detected in both MBL vs. HD (mainly upregulated) and CLL vs. HD (mostly downregulated). The last three pathways were also observed for MBL vs. CLL (upregulated in MBL).
To unravel the previous comparison, the MCL algorithm was used, and this disclosed 3 main clusters of non-redundant, upregulated proteins in MBL (Table 2). Cluster #1 involved pathways related to receptor binding (including growth factor receptors such as the ErbB family) and signal transduction, with an emphasis on the PI3K-Akt pathway. The activation of these pathways promotes cell survival and migration. Cluster #2 included cell division pathways and upregulated HMGB1 and MYC, both allowing gene transcription and proliferation. Cluster #3 concerned immune system activation, in particular inflammatory responses involving innate immune cells, viral interactions, IL17, and the JAK-STAT pathway, the latter playing a major role in cytokine receptor signaling.
Conclusions: The serum proteins in M-MBL suggest immune stimulation, which underwent considerable change after transition to M-CLL. This highlights an evolving serum proteome functionality with a slight activation of the immune system in MBL that declines upon transition to CLL. Clustering analysis identified 3 non-redundant, upregulated protein clusters in M-MBL compared to M-CLL. These findings suggest immune stimulation as a characteristic feature in MBL that is not operative in CLL and point out 3 groups of distinct pathological networks that may have a role in disease onset and tumor control. Future studies, focusing on specific cell subsets, will be performed to disclose the main cell orchestrators and their exact role within each cluster, to ultimately develop preemptive strategies.
Acknowledgments. GB was supported by Fundación Alfonso Martín Escudero.
Disclosures
Allen:BMS: Current equity holder in publicly-traded company; C4 Therapeutics: Current equity holder in private company; Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Research Funding. Barrientos:Merck, Oncternal, Pharmacyclics/Abbvie: Research Funding; Beigene, AstraZeneca, Pharmacyclics/Abbvie: Consultancy. Rhodes:Abbive: Consultancy; Beigene: Consultancy; Genentech: Consultancy; Genmab: Consultancy; Janssen: Consultancy, Research Funding; Morphosys: Consultancy; Pharmacyclics: Consultancy, Research Funding; SeaGen: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy; Oncternal: Research Funding; Velosbios: Research Funding; Loxo Oncology: Research Funding; Epizyme: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.